Skills to Develop
- To describe how electrons are grouped within atoms.
Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons occupy the space about the nucleus. Do they move around the nucleus at random, or do they exist in some ordered arrangement?
List Of Valence Electrons For Each Element
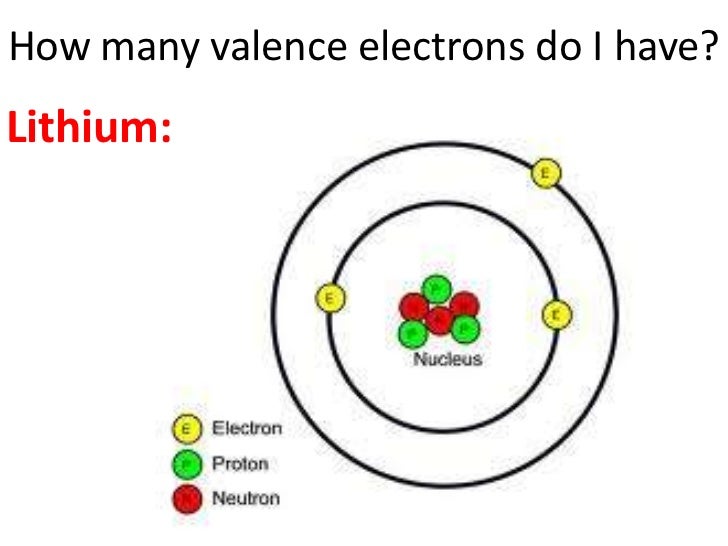
- Lithium is in group 1, or the first vertical column in the periodic table so it has 1 valence electron. I hope this helpe, and if you jave any further questions, feel free to ask.
- Across each row, or period, of the periodic table, the number of valence electrons in groups 1-2 and 13-18 increases by one from one element to the next. Within each column, or group, of the table, all the elements have the same number of valence electrons. Lithium has one valence electron.
Lithium has 1 valence electron and since it is a metal it looses its valence electrons to become stable. If it looses and electron it now has 3 protons and 2 electrons therefore there is 1 more.
The modern theory of electron behavior is called quantum mechanics. It makes the following statements about electrons in atoms:
- Electrons in atoms can have only certain specific energies. We say that the energies of the electrons are quantized.
- Electrons are organized according to their energies into sets called shells. Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. Shells do not have specific, fixed distances from the nucleus, but an electron in a higher-energy shell will spend more time farther from the nucleus than does an electron in a lower-energy shell.
- Shells are further divided into subsets of electrons called subshells. The first shell has only one subshell, the second shell has two subshells, the third shell has three subshells, and so on. The subshells of each shell are labeled, in order, with the letters s, p, d, and f. Thus, the first shell has only an s subshell, the second shell has an s and a p subshell, the third shell has s, p, and d subshells, and so forth.
- Different subshells hold a different maximum number of electrons. Any s subshell can hold up to 2 electrons; p supshell up to 6 electrons; d subshell up to 10; and f subshell up to 14.
It is the arrangement of electrons into shells that has the most effect on chemical properties, so we will focus on mainly on shells here.
We use numbers to indicate which shell an electron is in. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell (labeled 1s) and can hold a maximum of 2 electrons. This is why there are two elements in the first row of the periodic table (H & He).
Because the first shell can only hold a maximum of 2 electrons, the third electron must go into the second shell. Therefore the lithium (Li), which has three total electrons, will have two electrons in the first shell and one electron in the second shell. Kodi krypton android apk. Notice that lithium is the first element in the second row of the periodic table.
The second shell has two subshells (labeled 2s and 2p). The 2s subshell holds a maximum of 2 electrons, and the 2p subshell holds a maximum of 6 electrons. This means that the second shell can hold a maximum of eight electrons (2+6=8). Notice that there are eight elements in the second row of the periodic table.
It is only the electrons in the outer-most shell, called the VALENCE shell, that tend to react (be gained, lost, or shared). You might imagine that, if two atoms bumped into each other, it would be the outer electrons that would interact first. The following is a list of total electrons, electrons by shell, and valence electrons for the first 10 elements.
Number Of Electrons In Boron
- Hydrogen has 1 electron in the first shell (so one valence electron).
- Helium has 2 electrons --- both in the first shell (so two valence electrons).
- Lithium has 3 electrons --- 2 in the first shell, and 1 in the second shell (so one valence electron).
- Beryllim has 4 electrons --- 2 in the first shell, and 2 in the second shell (so two valence electrons).
- Boron has 5 electrons --- 2 in the first shell, and 3 in the second shell (so three valence electrons).
- Carbon has 6 electrons --- 2 in the first shell, and 4 in the second shell (so four valence electrons).
- Nitrogen has 7 electrons --- 2 in the first shell, and 5 in the second shell (so five valence electrons).
- Oxygen has 8 electrons --- 2 in the first shell, and 6 in the second shell (so six valence electrons).
- Fluorine has 9 electrons --- 2 in the first shell, and 7 in the second shell (so seven valence electrons).
- Neon has 10 electrons --- 2 in the first shell, and 8 in the second shell (so eight valence electrons).
Figure 2.6.1 below lists the atomic number for the main group elements. The atomic number defines the number of protons in the nucleus of each atom. For neutral atoms, the number of positive protons will equal the total number of negative electrons (zero net charge). For example, bromine (Br) has 35 protons and 35 total electrons. Periodic tables always list the atomic number.
Figure 2.6.1 - Atomic Number for Each of the Main Group Elements
The number of valence electrons for each main group element can be determined by the column, or group, it occupies on the periodic table. Table 2.6.2 below summarizes the number of valence electrons for each main group column of elements. For example, the elements in the first column (sometimes labeled IA), all have one valence electron. The second column (IIA) has two valence electrons. We skip the short block of ten elements in the middle because this is where a subshell fills out of order. The elements in columns IIIA, IVA, VA, VIA, and VIIA, and VIIIA* have three, four, five, six, seven, and eight* valence electrons, respectively.
*Note that helium (He) only has two valence electrons. Some periodic tables place helium in column IIA, others place it in VIIIA, and some in both locations.
Figure 2.6.2 - Number of Valence Electrons for Main Group Elements
Example (PageIndex{1}): ElectronS of Phosphorus Atoms
How many total and valence electrons are in a neutral phosphorus atom?
SOLUTION
A neutral phosphorus atom has 15 total electrons. Two electrons can go into first shell, eight in the second shell, and it has five more in the third shell. The third shell is the outer valence shell, so it has 5 valence electrons.
The number of electrons in each shell becomes more complicated as more electrons are added because there are more subshells being used and because the shell start to fill out of order. For elements with larger atomic number than 20 (beyond calcium), we will just focus on how many total and how many valence electrons, not the number in each shell. We have stated that the outer-shell electrons are called valence. The inner (non-valence) shells and electrons are often called the core.
Example (PageIndex{2}): Counting total and Valence Electrons in Xenon Atoms
How many total, valence, and core electrons are there in a neutral xenon atom?
SOLUTION
Xenon has 54 total, 8 valence, and 46 core electrons.
Concept Review Exercises
- How are electrons organized in atoms?
- What is the maximum number of electrons that can fit into the first two shells of an atom?
- What is the difference between core electrons and valence electrons?
Answers
- Electrons are organized into shells and subshells around nuclei.
- The first shell can fit a maximum of two and the second shell can fit a maximum of eight electrons.
- Valence electrons are in the highest-numbered (outer) shell; all other electrons are core electrons.
Key Takeaway
- Electrons are organized into shells and subshells about the nucleus of an atom.
Electron Configuration
The electrons in an atom fill up its atomic orbitals according to the Aufbau Principle; 'Aufbau,' in German, means 'building up.' The Aufbau Principle, which incorporates the Pauli Exclusion Principle and Hund's Rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals:
- Electrons always fill orbitals of lower energy first. 1s is filled before 2s, and 2s before 2p.
- The Pauli Exclusion Principle states no two electrons within a particular atom can have identical quantum numbers. In function, this principle means that if two electrons occupy the same orbital, they must have opposite spin.
- Hund's Rule states that when an electron joins an atom and has to choose between two or more orbitals of the same energy, the electron will prefer to enter an empty orbital rather than one already occupied. As more electrons are added to the atom, these electrons tend to half-fill orbitals of the same energy before pairing with existing electrons to fill orbitals.
Valency and Valence Electrons
The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Valence electrons are the highest energy electrons in an atom and are therefore the most reactive. While inner electrons (those not in the valence shell) typically don't participate in chemical bonding and reactions, valence electrons can be gained, lost, or shared to form chemical bonds. For this reason, elements with the same number of valence electrons tend to have similar chemical properties, since they tend to gain, lose, or share valence electrons in the same way. The Periodic Table was designed with this feature in mind. Each element has a number of valence electrons equal to its group number on the Periodic Table. This table illustrates a number of interesting, and complicating, features of electron configuration.
First, as electrons become higher in energy, a shift takes place. Up until now, we have said that as the principle quantum number, increases, so does the energy level of the orbital. And, as we stated above in the Aufbau principle, electrons fill lower energy orbitals before filling higher energy orbitals. However, the diagram above clearly shows that the 4s orbital is filled before the 3d orbital. In other words, once we get to principle quantum number 3, the highest subshells of the lower quantum numbers eclipse in energy the lowest subshells of higher quantum numbers: 3d is of higher energy than 4s.
Second, the above indicates a method of describing an element according to its electron configuration. As you move from left to right across the periodic table, the above diagram shows the order in which orbitals are filled. If we were the actually break down the above diagram into groups rather than the blocks we have, it would show how exactly how many electrons each element has. For example, the element of hydrogen, located in the uppermost left-hand corner of the periodic table, is described as 1s1, with the s describing which orbital contains electrons and the 1 describing how many electrons reside in that orbital. Lithium, which resides on the periodic table just below hydrogen, would be described as 1s22s1. The electron configurations of the first ten elements are shown below (note that the valence electrons are the electron in highest energy shell, not just the electrons in the highest energy subshell).
The Octet Rule
Shadow war armageddon rules. Our discussion of valence electron configurations leads us to one of the cardinal tenets of chemical bonding, the octet rule. The octet rule states that atoms becomeespecially stable when their valence shells gain a full complement of valence electrons. For example, in above, Helium (He) and Neon (Ne) have outer valence shells that are completely filled, so neither has a tendency to gain or lose electrons. Therefore, Helium and Neon, two of the so-called Noble gases, exist in free atomic form and do not usually form chemical bonds with other atoms.
Most elements, however, do not have a full outer shell and are too unstable to exist as free atoms. Instead they seek to fill their outer electron shells by forming chemical bonds with other atoms and thereby attain Noble Gas configuration. An element will tend to take the shortest path to achieving Noble Gas configuration, whether that means gaining or losing one electron. For example, sodium (Na), which has a single electron in its outer 3s orbital, can lose that electron to attain the electron configuration of neon. Chlorine, with seven valence electrons, can gain one electron to attain the configuration of argon. When two different elements have the same electron configuration, they are called isoelectronic. Ddt2000 software.
Diamagnetism and Paramagnetism
The electron configuration of an atom also has consequences on its behavior in relation to magnetic fields. Such behavior is dependent on the number of electrons an atom has that are spin paired. Remember that Hund's Rule and the Pauli Exclusion Principle combine to dictate that an atom's orbitals will all half-fill before beginning to completely fill, and that when they completely fill with two electrons, those two electrons will have opposite spins.
An atom with all of its orbitals filled, and therefore all of its electrons paired with an electron of opposite spin, will be very little affected by magnetic fields. Such atoms are called diagmetic. Conversely, paramagnetic atoms do not have all of their electrons spin-paired and are affected by magnetic fields. There are degrees of paramagnetism, since an atom might have one unpaired electron, or it might have four.
